HAVE ANY QUESTIONS? CLICK HERE
In'Oss
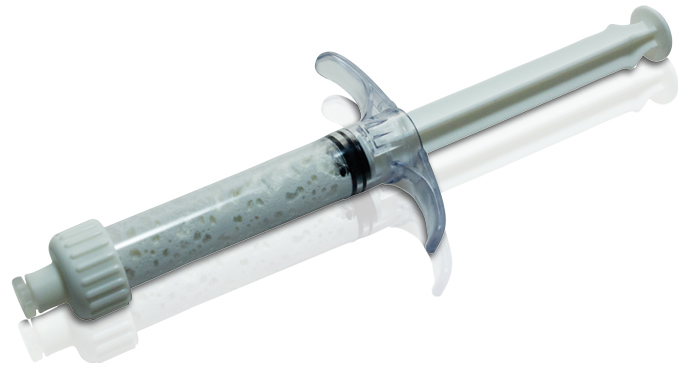
Injectable Bone Substitute
Moldable Bone Graft Substitute
Building on Biomatlante’s core MBCP® Technology, In’Oss™ is an innovative and moldable bone graft3,14,15, composed of Hydroxyapatite (HA), Beta Tricalcium Phosphate (ß-TCP) and a hydrogel12.
Developed to facilitate handling3,15 during bone grafting procedures, In’Oss™ can fit into different grafting sites.3,14,15
In’Oss™ keeps the original graft shape and bone volume3,14,15. It is gradually absorbed to be replaced by vital architectured bone.7,8,14
Developed to facilitate handling3,15 during bone grafting procedures, In’Oss™ can fit into different grafting sites.3,14,15
In’Oss™ keeps the original graft shape and bone volume3,14,15. It is gradually absorbed to be replaced by vital architectured bone.7,8,14

MBCP® Technology’s Key Features
Provides a matrix for new bone growth
Macropores are a network of interconnected spaces that promote the biological infiltration and cellular colonization by osteoblasts and osteoclasts.
Orthopaedics and Maxillofacial:
It is intended for use as a bone graft to fill or reconstruct osseous bone defects or gaps of the skeletal system (e.g. extremities, spine and pelvis, dental) that are not intrinsic to the stability of the bone structure. Osseous defects can occur as a result of a trauma or in surgically created defects.
It can be used with autograft as a bone graft extender.
It is intended for use as a bone graft to fill or reconstruct osseous bone defects or gaps of the skeletal system (e.g. extremities, spine and pelvis, dental) that are not intrinsic to the stability of the bone structure. Osseous defects can occur as a result of a trauma or in surgically created defects.
It can be used with autograft as a bone graft extender.
In’Oss is available in syringe of:
0.5 mL – 1 mL – 2.5 mL – 5 mL – 10 mL
0.5 mL – 1 mL – 2.5 mL – 5 mL – 10 mL
1] Dupraz – 1997 – “Ultrastructural study of long term implanted CaP particulate materials into rabbits bones” Bioceramics
[2] Weiss – 2007 – “The safety and efficacy of an injectable bone substitute in dental sockets demonstrated in a human clinical trial” Biomaterials.
[3] Daculsi – 2012 – “Development and clinical cases of injectable bone void filler used in orthopaedic” IRBM.
[4] Legeros – 2002 – “Properties of Osteoconductive Biomaterials: Calcium Phosphates” – Clinical Orthopedics
[5] Ransford – 1998 – “Synthetic porous ceramic compared with autograft in scoliosis surgery 341 patient randomised study” The Journal of Bone and Joint Surgery
[6] Daculsi – 2008 – “Effect of sintering process of HA/TCP bioceramics on microstructure, dissolution, cell proliferation and Bone ingrowth” Key Engineering Materials
[7] Weiss – 2005 – “A New Injectable Bone Substitute Concept (MBCP Gel TM): First Clinical Results in Human Maxillo-Facial Surgery ” Key Engineering Materials.
[8] Seris – 2013 – “Bone regeneration at the expense of Bioceramic/Hydrogel composite: clinical evidence” IRBM.
[9] Miramond – 2014 – “Comparative critical study of commercial calcium phosphate bone substitutes in termes of physico-chemical properties” – Key Engineering Materials
[10] Duan – 2017 – “Variation of bone forming ability with the physicochemical properties of calcium phosphate bone substitutes” – Article on line
[11] Clergeau – 2003 – “Osteoconductive potential of an injectable calcium phosphate in the prevention of alveolar crest resorption after extraction of mandibular molar or premolar teeth” Case study Report of Biomatlante
[12] Daculsi – 1998 – “Biphasic calcium phosphate concept applied to artificial bone, implant coating and injectable bone substitute
[13] Gouin – 1995 – “Biphasic macroporous calcium phosphate ceramine bone substitute for filling bone defects: a report of 23 cases” Revue de Chirurgie Orthopédique
[14] Daculsi – 2015 – “Clinical performance of moldable bioceramic for bone regeneration in maxillofacial surgery” Journal of biomimetics, biomaterials and biomedical Engineering
[15] Fabre – 2008 – “Pilot Study of Safety and Performance of a Mixture of Calcium Phosphate Granules Combined with Cellulosic-Derived Gel after Tunnel Filling Created during Surgical Treatment of Femoral Head Aseptic Osteonecrosis” Key Engineering Materials.
[16] Nery – 1992 – “Tissue response to biphasic calcium phosphate ceramic with different ratios of HA/TCP in periodontal osseous defects” – Journal of Periodontology
[17] Schaefer – 2011 – “How degradation of calcium phosphate bone substitute materials is influenced by phase composition and porosity” – Advanced Engineering Materials
[18] Daculsi – 2013 – “Clinical studies of anterior cervical fusion with PEEK cages: comparing illiac graft and a Macroporous Biphasic Calcium Phosphate
[19] Daculsi – 1999 – “Spongious and cortical bone substitution kinetics at the expense of macroporous biphasic calcium phosphate : animal and human evidence” Bioceramics
[20] Gauthier – 1998 – “Macroporous biphasic calcium phosphate ceramics: influence of macropore diameter and macroporosity percentage on bone ingrowth” Biomaterials
[21] Legeros – 1988 – “Significance of the porosity and physical chemistry of Calcium Phosphate Ceramics Biodegradation-Bioresorption
[2] Weiss – 2007 – “The safety and efficacy of an injectable bone substitute in dental sockets demonstrated in a human clinical trial” Biomaterials.
[3] Daculsi – 2012 – “Development and clinical cases of injectable bone void filler used in orthopaedic” IRBM.
[4] Legeros – 2002 – “Properties of Osteoconductive Biomaterials: Calcium Phosphates” – Clinical Orthopedics
[5] Ransford – 1998 – “Synthetic porous ceramic compared with autograft in scoliosis surgery 341 patient randomised study” The Journal of Bone and Joint Surgery
[6] Daculsi – 2008 – “Effect of sintering process of HA/TCP bioceramics on microstructure, dissolution, cell proliferation and Bone ingrowth” Key Engineering Materials
[7] Weiss – 2005 – “A New Injectable Bone Substitute Concept (MBCP Gel TM): First Clinical Results in Human Maxillo-Facial Surgery ” Key Engineering Materials.
[8] Seris – 2013 – “Bone regeneration at the expense of Bioceramic/Hydrogel composite: clinical evidence” IRBM.
[9] Miramond – 2014 – “Comparative critical study of commercial calcium phosphate bone substitutes in termes of physico-chemical properties” – Key Engineering Materials
[10] Duan – 2017 – “Variation of bone forming ability with the physicochemical properties of calcium phosphate bone substitutes” – Article on line
[11] Clergeau – 2003 – “Osteoconductive potential of an injectable calcium phosphate in the prevention of alveolar crest resorption after extraction of mandibular molar or premolar teeth” Case study Report of Biomatlante
[12] Daculsi – 1998 – “Biphasic calcium phosphate concept applied to artificial bone, implant coating and injectable bone substitute
[13] Gouin – 1995 – “Biphasic macroporous calcium phosphate ceramine bone substitute for filling bone defects: a report of 23 cases” Revue de Chirurgie Orthopédique
[14] Daculsi – 2015 – “Clinical performance of moldable bioceramic for bone regeneration in maxillofacial surgery” Journal of biomimetics, biomaterials and biomedical Engineering
[15] Fabre – 2008 – “Pilot Study of Safety and Performance of a Mixture of Calcium Phosphate Granules Combined with Cellulosic-Derived Gel after Tunnel Filling Created during Surgical Treatment of Femoral Head Aseptic Osteonecrosis” Key Engineering Materials.
[16] Nery – 1992 – “Tissue response to biphasic calcium phosphate ceramic with different ratios of HA/TCP in periodontal osseous defects” – Journal of Periodontology
[17] Schaefer – 2011 – “How degradation of calcium phosphate bone substitute materials is influenced by phase composition and porosity” – Advanced Engineering Materials
[18] Daculsi – 2013 – “Clinical studies of anterior cervical fusion with PEEK cages: comparing illiac graft and a Macroporous Biphasic Calcium Phosphate
[19] Daculsi – 1999 – “Spongious and cortical bone substitution kinetics at the expense of macroporous biphasic calcium phosphate : animal and human evidence” Bioceramics
[20] Gauthier – 1998 – “Macroporous biphasic calcium phosphate ceramics: influence of macropore diameter and macroporosity percentage on bone ingrowth” Biomaterials
[21] Legeros – 1988 – “Significance of the porosity and physical chemistry of Calcium Phosphate Ceramics Biodegradation-Bioresorption
To read the complete precautions, please refer to the Intructions For Use.
The product must only be handled or implanted by trained qualified physicians having read the instructions for use.
In'Oss™ Putty is intended for use by surgeons familiar with bone grafting and rigid fixation techniques.
The product must only be handled or implanted by trained qualified physicians having read the instructions for use.
In'Oss™ Putty is intended for use by surgeons familiar with bone grafting and rigid fixation techniques.
- ISO 13485:2016 MDSAP
- Read the instructions for use
- MBCP® Putty is supplied sterile and CE-marked as a Class III Medical Device according to Directive EEC/93/42

Not all products are registered or available in every country/region. Please check with Biomatlante representative for availability and further information.